Overexpression of the HER2 protein occurs in approximately 25% of breast cancer cases, resulting in a therapeutic target of great interest against which various anti-HER2 therapies against breast cancer are being developed.
In this post we summarize the main types of anti HER2 therapies that are being incorporated into clinical practice in the approach to breast cancer.
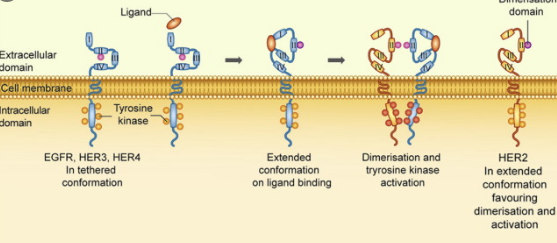
The HER2 protein, also known as ERBB-2, is a 185 kDa tyrosine kinase receptor composed of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain with tyrosine kinase activity.
By binding to EGF (Epidermal Growth Factor) ligands, such as TGF-α , AR, EPR or NRG1 , among others, the formation of heterodimers is activated that activate various transduction cascades, resulting in increased proliferation, differentiation, cell mobility and survival.
Overexpression of this receptor and its constant activation leads to uncontrolled cell division and carcinogenesis.
After focusing on the HER2 receptor as a therapeutic target, various treatments aimed at blocking and deactivating it have been developed as a mechanism to treat HER2 + breast cancer.
There are currently three types of anti-HER2 therapies for breast cancer : monoclonal antibodies, TKIs (tyrosine kinase inhibitors) and ADCs (Antibody-drug conjugates).
MONOCLONAL ANTIBODIES (MAB)
Anti HER2 monoclonal antibodies are large molecules with monovalent affinity, produced using recombinant DNA technology.
Its mechanism of action is based on blocking the extracellular ligand binding domain of the HER2 receptor, thus reducing the neoplastic actions of HER2 heterodimers. At the same time, the HER2-antibody complex is internalized and destroyed by the target cell, reducing the number of available HER2 receptors.
Examples of monoclonal antibodies currently used as treatment in breast cancer include humanized antibodies trastuzumab and pertuzumab.
You can expand the information in this entry about Monoclonal Antibodies against breast cancer .
TYROSINE KINASE INHIBITORS (TKI)
Tyrosine kinase inhibitors are smaller molecules than monoclonal anti-HER2 antibodies, and their mechanism of action is based on the inhibition of phosphorylation of the intracellular tyrosine kinase domain of the HER2 receptor.
Most of these compounds target more than one HER receptor, allowing them to simultaneously block two or more components of the heterodimer.
The binding of these compounds to the HER2 receptor can be reversible as in the case of Lapatinib , or irreversible as in the case of Canertinib .
ANTIBODY-DRUG CONJUGATES (ADC)
ADCs are a special type of therapeutic antibody, consisting of an anti-HER2 monoclonal antibody conjugated to a chemotherapeutic drug. Its mechanism of action is based on the internalization of the antibody-drug complex and its intracellular cytotoxic activity.
The selection of the antigen for the development of this type of therapeutic agents is critical to achieve maximum specificity and consequently less toxicity, with optimal antigens being those that are expressed with high density on the surface of cancer cells, and do not do so in healthy cells.
An example of ADC against breast cancer would be trastuzumab emtansine (T-DM1).